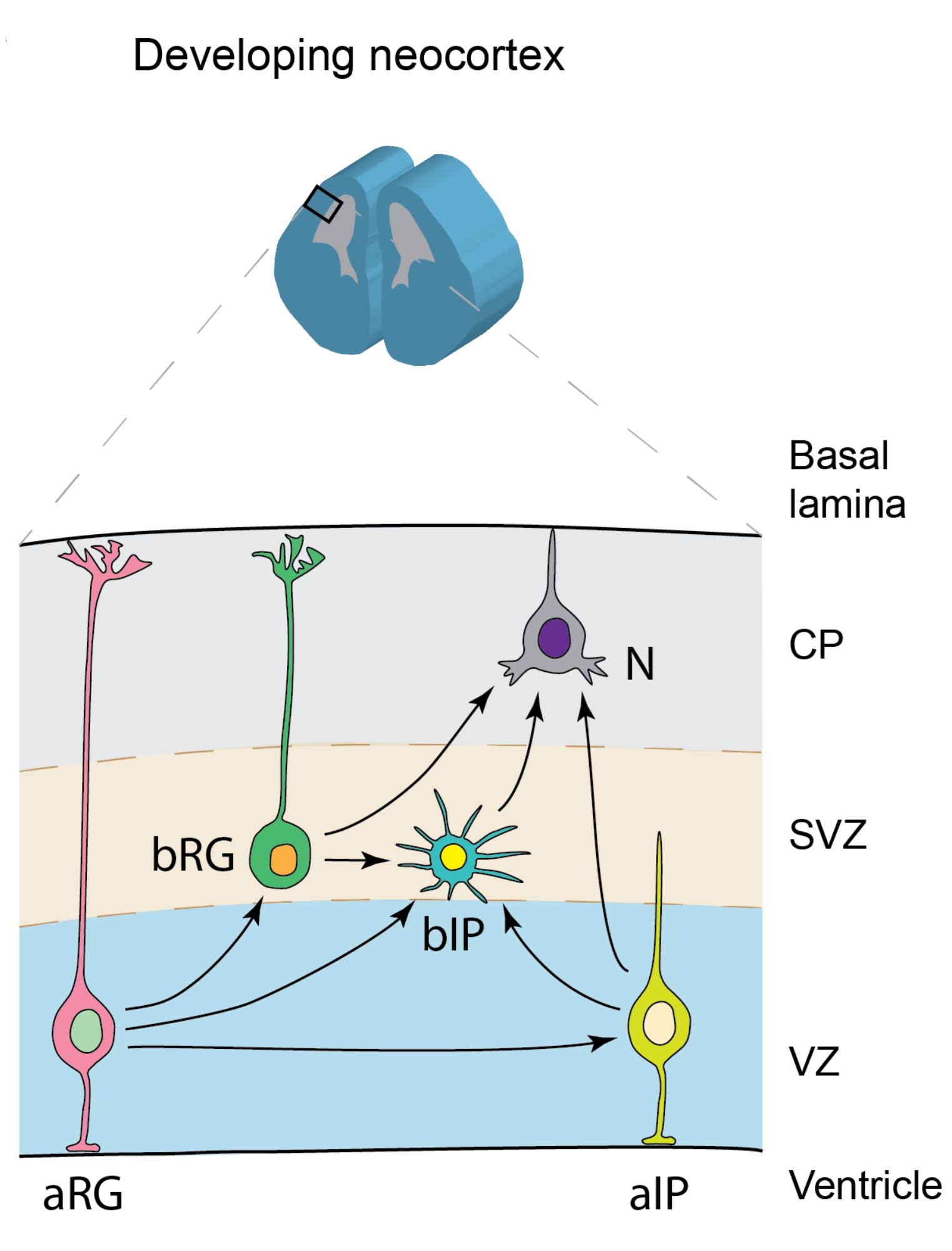
How do neural stem cells contribute to neocortex development?
In this review article, we discuss the great diversity of neural stem cells (NSCs) and their contribution to the development of the neocortex. First, we review different classes and types of NSCs based on their cell biological features, and discuss recent advances in characterizing their marker expression and cell polarity features. Second, we review the different modes of cell divisions that NPCs undergo and discuss the importance of the balance between proliferation and differentiation of NPCs in neocortical development. Third, we review the different proliferative capacities among individual NPC types in different mammalian species.

Does a bigger brain necessarily confer enhanced cognitive abilities?
During human evolution, the neocortex, the evolutionarily youngest part of the cerebral cortex, expanded dramatically and made the human brain larger. The expression of human-specific gene ARHGAP11B in non-human species was found to lead to an increased neural stem cell number and an expanded neocortex in mouse embryos, ferret pups, and common marmoset fetuses. In this study, we show that mice expressing the human-specific gene ARHGAP11B develop a bigger brain with more upper-layer neurons, which persist into adulthood, and show increased memory flexibility and reduced anxiety.

Happiness and the evolution of brain size
Serotonin is often called the happiness neurotransmitter because the function of serotonin in the adult brain is associated with creating the feeling of well-being. During development, the placenta produces serotonin, which then reaches the brain via the blood circulation. Our study uncovers a novel role of serotonin as a growth factor to promote neural stem cell proliferation, through binding to serotonin receptor 2a and HER2/ERK signaling pathway. Therefore, serotonin emerges as an important extrinsic pro-proliferative signal for neural stem cells, which may have contributed to evolutionary expansion of the human neocortex.
Publication list
2024
Xing L; Huttner W B; Namba T
Role of cell metabolism in the pathophysiology of brain size-associated neurodevelopmental disorders
In: Neurobiology of Disease, vol. 199, pp. 106607, 2024, ISSN: 0969-9961.
@article{XING2024106607,
title = {Role of cell metabolism in the pathophysiology of brain size-associated neurodevelopmental disorders},
author = {Lei Xing and Wieland B. Huttner and Takashi Namba},
url = {https://www.sciencedirect.com/science/article/pii/S0969996124002079},
doi = {10.1016/j.nbd.2024.106607},
issn = {0969-9961},
year = {2024},
date = {2024-07-22},
urldate = {2024-01-01},
journal = {Neurobiology of Disease},
volume = {199},
pages = {106607},
abstract = {Cell metabolism is a key regulator of human neocortex development and evolution. Several lines of evidence indicate that alterations in neural stem/progenitor cell (NPC) metabolism lead to abnormal brain development, particularly brain size-associated neurodevelopmental disorders, such as microcephaly. Abnormal NPC metabolism causes impaired cell proliferation and thus insufficient expansion of NPCs for neurogenesis. Therefore, the production of neurons, which is a major determinant of brain size, is decreased and the size of the brain, especially the size of the neocortex, is significantly reduced. This review discusses recent progress understanding NPC metabolism, focusing in particular on glucose metabolism, fatty acid metabolism and amino acid metabolism (e.g., glutaminolysis and serine metabolism). We provide an overview of the contributions of these metabolic pathways to brain development and evolution, as well as to the etiology of neurodevelopmental disorders. Furthermore, we discuss the advantages and disadvantages of various experimental models to study cell metabolism in the developing brain.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xing L; Gkini V; Nieminen A I; Zhou H; Aquilino M; Naumann R; Reppe K; Tanaka K; Carmeliet P; Heikinheimo O; Pääbo S; Huttner W B; Namba T
In: Nature Communications, vol. 15, iss. 1, pp. 3468, 2024, ISSN: 2041-1723.
@article{Xing2024,
title = {Functional synergy of a human-specific and an ape-specific metabolic regulator in human neocortex development},
author = {Lei Xing and Vasiliki Gkini and Anni I. Nieminen and Hui-Chao Zhou and Matilde Aquilino and Ronald Naumann and Katrin Reppe and Kohichi Tanaka and Peter Carmeliet and Oskari Heikinheimo and Svante Pääbo and Wieland B. Huttner and Takashi Namba},
doi = {10.1038/s41467-024-47437-8},
issn = {2041-1723},
year = {2024},
date = {2024-04-24},
urldate = {2024-04-24},
journal = {Nature Communications},
volume = {15},
issue = {1},
pages = {3468},
publisher = {Springer Science and Business Media LLC},
abstract = {Metabolism has recently emerged as a major target of genes implicated in the evolutionary expansion of human neocortex. One such gene is the human-specific gene ARHGAP11B. During human neocortex development, ARHGAP11B increases the abundance of basal radial glia, key progenitors for neocortex expansion, by stimulating glutaminolysis (glutamine-to-glutamate-to-alpha-ketoglutarate) in mitochondria. Here we show that the ape-specific protein GLUD2 (glutamate dehydrogenase 2), which also operates in mitochondria and converts glutamate-to-αKG, enhances ARHGAP11B’s ability to increase basal radial glia abundance. ARHGAP11B + GLUD2 double-transgenic bRG show increased production of aspartate, a metabolite essential for cell proliferation, from glutamate via alpha-ketoglutarate and the TCA cycle. Hence, during human evolution, a human-specific gene exploited the existence of another gene that emerged during ape evolution, to increase, via concerted changes in metabolism, progenitor abundance and neocortex size.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2023
Luppi A I; Girn M; Rosas F E; Timmermann C; Roseman L; Erritzoe D; Nutt D J; Stamatakis E A; Spreng R N; Xing L; Huttner W B; Carhart-Harris R L
A role for the serotonin 2A receptor in the expansion and functioning of human transmodal cortex
In: Brain, pp. awad311, 2023, ISSN: 0006-8950.
@article{pmid37703310,
title = {A role for the serotonin 2A receptor in the expansion and functioning of human transmodal cortex},
author = {Andrea I Luppi and Manesh Girn and Fernando E Rosas and Christopher Timmermann and Leor Roseman and David Erritzoe and David J Nutt and Emmanuel A Stamatakis and R Nathan Spreng and Lei Xing and Wieland B Huttner and Robin L Carhart-Harris},
doi = {10.1093/brain/awad311},
issn = {0006-8950},
year = {2023},
date = {2023-09-01},
urldate = {2023-09-01},
journal = {Brain},
pages = {awad311},
abstract = {Integrating independent but converging lines of research on brain function and neurodevelopment across scales, this article proposes that serotonin 2A receptor (5-HT2AR) signaling is an evolutionary and developmental driver and potent modulator of the macroscale functional organization of the human cerebral cortex. A wealth of evidence indicates that the anatomical and functional organization of the cortex follows a unimodal-to-transmodal gradient. Situated at the apex of this processing hierarchy - where it plays a central role in the integrative processes underpinning complex, human-defining cognition - the transmodal cortex has disproportionately expanded across human development and evolution. Notably, the adult human transmodal cortex is especially rich in 5-HT2AR expression, and recent evidence suggests that, during early brain development, 5-HT2AR signaling on neural progenitor cells stimulates their proliferation - a critical process for evolutionarily-relevant cortical expansion. Drawing on multimodal neuroimaging and cross-species investigations, we argue that, by contributing to the expansion of the human cortex, and being prevalent at the apex of its hierarchy in the adult brain, 5-HT2AR signaling plays a major role in both human cortical expansion and functioning. Due to its unique excitatory and downstream cellular effects, neuronal 5-HT2AR agonism promotes neuroplasticity, learning, and cognitive and psychological flexibility in a context-(hyper)sensitive manner with therapeutic potential. Overall, we delineate a dual role of 5-HT2ARs in enabling both the expansion and modulation of the human transmodal cortex.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xing L; Pinson A; Mora-Bermúdez F; Huttner W B
In: Neocortical Neurogenesis in Development and Evolution, Chapter 8, pp. 137-156, John Wiley & Sons, Ltd, 2023, ISBN: 9781119860914.
@inbook{doi:https://doi.org/10.1002/9781119860914.ch8,
title = {The Role of Human-specific Genes and Amino Acid Substitutions for Neocortex Expansion and Modern Human vs. Neanderthal Differences in Neocortical Neurogenesis},
author = { Lei Xing and Anneline Pinson and Felipe Mora-Bermúdez and Wieland B Huttner},
url = {https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119860914.ch8},
doi = {https://doi.org/10.1002/9781119860914.ch8},
isbn = {9781119860914},
year = {2023},
date = {2023-01-01},
booktitle = {Neocortical Neurogenesis in Development and Evolution},
pages = {137-156},
publisher = {John Wiley & Sons, Ltd},
chapter = {8},
abstract = {The neural stem and progenitor cells (NSPCs) in the developing neocortex have a key role in its expansion and in neocortical neurogenesis. Here, we summarize the key features of the two principal classes of neocortical NSPCs, the apical progenitors (APs) residing in the ventricular zone (VZ) and the basal progenitors (BPs) residing in the subventricular zone (SVZ). We explain how the differences in the cell biology of BPs vs. APs endow the former class of NSPCs with a crucial advantage for neocortex expansion in development and evolution. Next, we discuss the human-specific gene ARHGAP11B and its ability to amplify BPs, which is based on the localization of the ARHGAP11B protein in mitochondria and its stimulation of the metabolic pathway glutaminolysis, eventually resulting in increased neocortical neurogenesis and neocortex expansion. Finally, we address the crucial roles of modern human-specific amino acid substitutions in proteins that govern fundamental aspects of neocortical development. Thus, three such substitutions in KIF18a and KNL1 reduce the rate of chromosome segregation errors upon AP mitosis, implying less of these errors in the radial units of modern human than Neanderthal neocortex. Furthermore, a single modern human-specific amino acid substitution in transketolase-like 1 (TKTL1) underlies its ability to selectively amplify basal radial glia, the BP type generating most neocortical neurons. As a result, TKTL1 is implicated in greater neocortical neurogenesis in modern humans than Neanderthals, notably in the frontal lobe.},
keywords = {},
pubstate = {published},
tppubtype = {inbook}
}
2022
Pinson A; Xing L; Namba T; Kalebic N; Peters J; Oegema C E; Traikov S; Reppe K; Riesenberg S; Maricic T; Derihaci R; Wimberger P; Pääbo S; Huttner W B
Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals
In: Science, vol. 377, no. 6611, pp. eabl6422, 2022, ISSN: 1095-9203.
@article{pmid36074851,
title = {Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals},
author = {Anneline Pinson and Lei Xing and Takashi Namba and Nereo Kalebic and Jula Peters and Christina Eugster Oegema and Sofia Traikov and Katrin Reppe and Stephan Riesenberg and Tomislav Maricic and Razvan Derihaci and Pauline Wimberger and Svante Pääbo and Wieland B Huttner},
doi = {10.1126/science.abl6422},
issn = {1095-9203},
year = {2022},
date = {2022-09-01},
urldate = {2022-09-01},
journal = {Science},
volume = {377},
number = {6611},
pages = {eabl6422},
abstract = {Neanderthal brains were similar in size to those of modern humans. We sought to investigate potential differences in neurogenesis during neocortex development. Modern human transketolase-like 1 (TKTL1) differs from Neanderthal TKTL1 by a lysine-to-arginine amino acid substitution. Using overexpression in developing mouse and ferret neocortex, knockout in fetal human neocortical tissue, and genome-edited cerebral organoids, we found that the modern human variant, hTKTL1, but not the Neanderthal variant, increases the abundance of basal radial glia (bRG) but not that of intermediate progenitors (bIPs). bRG generate more neocortical neurons than bIPs. The hTKTL1 effect requires the pentose phosphate pathway and fatty acid synthesis. Inhibition of these metabolic pathways reduces bRG abundance in fetal human neocortical tissue. Our data suggest that neocortical neurogenesis in modern humans differs from that in Neanderthals.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mora-Bermúdez F; Kanis P; Macak D; Peters J; Naumann R; Xing L; Sarov M; Winkler S; Oegema C E; Haffner C; Wimberger P; Riesenberg S; Maricic T; Huttner W B; Pääbo S
In: Sci Adv, vol. 8, no. 30, pp. eabn7702, 2022, ISSN: 2375-2548.
@article{pmid35905187,
title = {Longer metaphase and fewer chromosome segregation errors in modern human than Neanderthal brain development},
author = {Felipe Mora-Bermúdez and Philipp Kanis and Dominik Macak and Jula Peters and Ronald Naumann and Lei Xing and Mihail Sarov and Sylke Winkler and Christina Eugster Oegema and Christiane Haffner and Pauline Wimberger and Stephan Riesenberg and Tomislav Maricic and Wieland B Huttner and Svante Pääbo},
doi = {10.1126/sciadv.abn7702},
issn = {2375-2548},
year = {2022},
date = {2022-07-01},
urldate = {2022-07-01},
journal = {Sci Adv},
volume = {8},
number = {30},
pages = {eabn7702},
abstract = {Since the ancestors of modern humans separated from those of Neanderthals, around 100 amino acid substitutions spread to essentially all modern humans. The biological significance of these changes is largely unknown. Here, we examine all six such amino acid substitutions in three proteins known to have key roles in kinetochore function and chromosome segregation and to be highly expressed in the stem cells of the developing neocortex. When we introduce these modern human-specific substitutions in mice, three substitutions in two of these proteins, KIF18a and KNL1, cause metaphase prolongation and fewer chromosome segregation errors in apical progenitors of the developing neocortex. Conversely, the ancestral substitutions cause shorter metaphase length and more chromosome segregation errors in human brain organoids, similar to what we find in chimpanzee organoids. These results imply that the fidelity of chromosome segregation during neocortex development improved in modern humans after their divergence from Neanderthals.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2021
Xing L; Wilsch-Bräuninger M; Huttner W B
How neural stem cells contribute to neocortex development
In: Biochem Soc Trans, vol. 49, no. 5, pp. 1997–2006, 2021, ISSN: 1470-8752.
@article{Xing_F_99,
title = {How neural stem cells contribute to neocortex development},
author = {Lei Xing and Michaela Wilsch-Bräuninger and Wieland B Huttner},
doi = {10.1042/BST20200923},
issn = {1470-8752},
year = {2021},
date = {2021-11-01},
urldate = {2021-11-01},
journal = {Biochem Soc Trans},
volume = {49},
number = {5},
pages = {1997--2006},
abstract = {The mammalian neocortex is the seat of higher cognitive functions, such as thinking and language in human. A hallmark of the neocortex are the cortical neurons, which are generated from divisions of neural progenitor cells (NPCs) during development, and which constitute a key feature of the well-organized layered structure of the neocortex. Proper formation of neocortex structure requires an orchestrated cellular behavior of different cortical NPCs during development, especially during the process of cortical neurogenesis. Here, we review the great diversity of NPCs and their contribution to the development of the neocortex. First, we review the categorization of NPCs into different classes and types based on their cell biological features, and discuss recent advances in characterizing marker expression and cell polarity features in the different types of NPCs. Second, we review the different modes of cell divisions that NPCs undergo and discuss the importance of the balance between proliferation and differentiation of NPCs in neocortical development. Third, we review the different proliferative capacities among different NPC types and among the same type of NPC in different mammalian species. Dissecting the differences between NPC types and differences among mammalian species is beneficial to further understand the development and the evolutionary expansion of the neocortex and may open up new therapeutic avenues for neurodevelopmental and psychiatric disorders.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xing L; Kubik-Zahorodna A; Namba T; Pinson A; Florio M; Prochazka J; Sarov M; Sedlacek R; Huttner W B
In: EMBO J, vol. 40, no. 13, pp. e107093, 2021, ISSN: 1460-2075.
@article{Xing_F_00,
title = {Expression of human-specific ARHGAP11B in mice leads to neocortex expansion and increased memory flexibility},
author = {Lei Xing and Agnieszka Kubik-Zahorodna and Takashi Namba and Anneline Pinson and Marta Florio and Jan Prochazka and Mihail Sarov and Radislav Sedlacek and Wieland B Huttner},
doi = {10.15252/embj.2020107093},
issn = {1460-2075},
year = {2021},
date = {2021-07-01},
urldate = {2021-07-01},
journal = {EMBO J},
volume = {40},
number = {13},
pages = {e107093},
abstract = {Neocortex expansion during human evolution provides a basis for our enhanced cognitive abilities. Yet, which genes implicated in neocortex expansion are actually responsible for higher cognitive abilities is unknown. The expression of human-specific ARHGAP11B in embryonic/foetal mouse, ferret and marmoset neocortex was previously found to promote basal progenitor proliferation, upper-layer neuron generation and neocortex expansion during development, features commonly thought to contribute to increased cognitive abilities. However, a key question is whether this phenotype persists into adulthood and if so, whether cognitive abilities are indeed increased. Here, we generated a transgenic mouse line with physiological ARHGAP11B expression that exhibits increased neocortical size and upper-layer neuron numbers persisting into adulthood. Adult ARHGAP11B-transgenic mice showed altered neurobehaviour, notably increased memory flexibility and a reduced anxiety level. Our data are consistent with the notion that neocortex expansion by ARHGAP11B, a gene implicated in human evolution, underlies some of the altered neurobehavioural features observed in the transgenic mice, such as the increased memory flexibility, a neocortex-associated trait, with implications for the increase in cognitive abilities during human evolution.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Souders C L; Wei C; Schmidt J T; Fonte D F D; Xing L; Trudeau V L; Martyniuk C J
In: Comp Biochem Physiol C Toxicol Pharmacol, vol. 243, pp. 108995, 2021, ISSN: 1532-0456.
@article{pmid33545344,
title = {Mitochondria of teleost radial glia: A novel target of neuroendocrine disruption by environmental chemicals?},
author = {Christopher L Souders and Chi Wei and Jordan T Schmidt and Dillon F Da Fonte and Lei Xing and Vance L Trudeau and Christopher J Martyniuk},
doi = {10.1016/j.cbpc.2021.108995},
issn = {1532-0456},
year = {2021},
date = {2021-05-01},
journal = {Comp Biochem Physiol C Toxicol Pharmacol},
volume = {243},
pages = {108995},
abstract = {In teleost fish, radial glial cells (RGCs) are progenitor cells for neurons and the major cell type synthesizing neuroestrogens. We hypothesized that chemical exposure impairs mitochondrial bioenergetics of RGCs, which then may lead to downstream consequences for neuroestrogen production. Here we provide proof of concept that mitochondria of RGCs can be perturbed by fungicides. We isolated RGCs from a mixed sex population of goldfish (Carassius auratus) and measured metabolic capacity of primary cells to a model mitotoxin fluazinam, a broad-spectrum fungicide that inhibits mitochondria electron transport chain (or ETC) Complex I. Using immunocytochemistry and real-time PCR, we demonstrate that the goldfish primary cell cultures are highly enriched for glia after multiple passages. Cytotoxicity assays revealed that glia treated with >25 μM fluazinam for 24 and 48-h showed reduced viability. As such, metabolic assays were conducted with non-cytotoxic concentrations (0.25-12.5 μM). Fluazinam did not affect oxygen consumption rates of RGCs at 24 h, but after 48 h, oligomycin induced ATP-linked respiration was decreased by both 6.25 and 12.5 μM fluazinam. Moreover, concentrations as low as 0.25 μM disrupted the mitochondrial membrane potential of RGCs, reflecting strong uncoupling effects of the fungicide on mitochondria. Here we provide proof of concept that mitochondrial bioenergetics of teleostean RGCs can be responsive to agrochemicals. Additional studies are required to address low-dose exposures in vivo and to determine if metabolic disruption impairs neuroendocrine functions of RGCs. We propose this mechanism constitutes a novel aspect of neuroendocrine disruption, significant because dysregulation of neuron-glia communication is expected to contribute to neuroendocrine disruption.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2020
Xing L; Kalebic N; Namba T; Vaid S; Wimberger P; Huttner W B
In: Neuron, vol. 108, no. 6, pp. 1113–1129.e6, 2020, ISSN: 1097-4199.
@article{Xing_F_50,
title = {Serotonin Receptor 2A Activation Promotes Evolutionarily Relevant Basal Progenitor Proliferation in the Developing Neocortex},
author = {Lei Xing and Nereo Kalebic and Takashi Namba and Samir Vaid and Pauline Wimberger and Wieland B Huttner},
doi = {10.1016/j.neuron.2020.09.034},
issn = {1097-4199},
year = {2020},
date = {2020-12-01},
urldate = {2020-12-01},
journal = {Neuron},
volume = {108},
number = {6},
pages = {1113--1129.e6},
abstract = {Evolutionary expansion of the mammalian neocortex (Ncx) has been linked to increased abundance and proliferative capacity of basal progenitors (BPs) in the subventricular zone during development. BP proliferation is governed by both intrinsic and extrinsic signals, several of which have been identified. However, a role of neurotransmitters, a canonical class of extrinsic signaling molecules, in BP proliferation remains to be established. Here, we show that serotonin (5-HT), via its receptor HTR2A, promotes BP proliferation in an evolutionarily relevant manner. HTR2A is not expressed in embryonic mouse Ncx; accordingly, 5-HT does not increase mouse BP proliferation. However, ectopic HTR2A expression can increase mouse BP proliferation. Conversely, CRISPR/Cas9-mediated knockout of endogenous HTR2A in embryonic ferret Ncx reduces BP proliferation. Pharmacological activation of endogenous HTR2A in fetal human Ncx ex vivo increases BP proliferation via HER2/ERK signaling. Hence, 5-HT emerges as an important extrinsic pro-proliferative signal for BPs, which may have contributed to evolutionary Ncx expansion.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Namba T; Dóczi J; Pinson A; Xing L; Kalebic N; Wilsch-Bräuninger M; Long K R; Vaid S; Lauer J; Bogdanova A; Borgonovo B; Shevchenko A; Keller P; Drechsel D; Kurzchalia T; Wimberger P; Chinopoulos C; Huttner W B
Human-Specific ARHGAP11B Acts in Mitochondria to Expand Neocortical Progenitors by Glutaminolysis
In: Neuron, vol. 105, no. 5, pp. 867–881.e9, 2020, ISSN: 1097-4199.
@article{pmid31883789,
title = {Human-Specific ARHGAP11B Acts in Mitochondria to Expand Neocortical Progenitors by Glutaminolysis},
author = {Takashi Namba and Judit Dóczi and Anneline Pinson and Lei Xing and Nereo Kalebic and Michaela Wilsch-Bräuninger and Katherine R Long and Samir Vaid and Janelle Lauer and Aliona Bogdanova and Barbara Borgonovo and Anna Shevchenko and Patrick Keller and David Drechsel and Teymuras Kurzchalia and Pauline Wimberger and Christos Chinopoulos and Wieland B Huttner},
doi = {10.1016/j.neuron.2019.11.027},
issn = {1097-4199},
year = {2020},
date = {2020-03-01},
journal = {Neuron},
volume = {105},
number = {5},
pages = {867--881.e9},
abstract = {The human-specific gene ARHGAP11B is preferentially expressed in neural progenitors of fetal human neocortex and increases abundance and proliferation of basal progenitors (BPs), which have a key role in neocortex expansion. ARHGAP11B has therefore been implicated in the evolutionary expansion of the human neocortex, but its mode of action has been unknown. Here, we show that ARHGAP11B is imported into mitochondria, where it interacts with the adenine nucleotide translocase (ANT) and inhibits the mitochondrial permeability transition pore (mPTP). BP expansion by ARHGAP11B requires its presence in mitochondria, and pharmacological inhibition of ANT function or mPTP opening mimic BP expansion by ARHGAP11B. Searching for the underlying metabolic basis, we find that BP expansion by ARHGAP11B requires glutaminolysis, the conversion of glutamine to glutamate for the tricarboxylic acid (TCA) cycle. Hence, an ARHGAP11B-induced, mitochondria-based effect on BP metabolism that is a hallmark of highly mitotically active cells appears to underlie its role in neocortex expansion.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xing L; Huttner W B
In: Front Cell Dev Biol, vol. 8, pp. 391, 2020, ISSN: 2296-634X.
@article{pmid32528958,
title = {Neurotransmitters as Modulators of Neural Progenitor Cell Proliferation During Mammalian Neocortex Development},
author = {Lei Xing and Wieland B Huttner},
doi = {10.3389/fcell.2020.00391},
issn = {2296-634X},
year = {2020},
date = {2020-01-01},
journal = {Front Cell Dev Biol},
volume = {8},
pages = {391},
abstract = {Neural progenitor cells (NPCs) play a central role during the development and evolution of the mammalian neocortex. Precise temporal and spatial control of NPC proliferation by a concert of cell-intrinsic and cell-extrinsic factors is essential for the correct formation and proper function of the neocortex. In this review, we focus on the regulation of NPC proliferation by neurotransmitters, which act as a group of cell-extrinsic factors during mammalian neocortex development. We first summarize, from both and studies, our current knowledge on how γ-aminobutyric acid (GABA), glutamate and serotonin modulate NPC proliferation in the developing neocortex and the potential involvements of different receptors in the underlying mechanisms. Another focus of this review is to discuss future perspectives using conditionally gene-modified mice and human brain organoids as model systems to further our understanding on the contribution of neurotransmitters to the development of a normal neocortex, as well as how dysregulated neurotransmitter signaling leads to developmental and psychiatric disorders.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2019
Gutierrez-Villagomez J M; Martyniuk C J; Xing L; Langlois V S; Pauli B D; Blais J M; Trudeau V L
In: Frontiers in Marine Science, vol. 6, pp. 533, 2019, ISSN: 2296-7745.
@article{10.3389/fmars.2019.00533,
title = {Transcriptome Analysis Reveals That Naphthenic Acids Perturb Gene Networks Related to Metabolic Processes, Membrane Integrity, and Gut Function in Silurana (Xenopus) tropicalis Embryos},
author = {Juan Manuel Gutierrez-Villagomez and Christopher J. Martyniuk and Lei Xing and Valerie S. Langlois and Bruce D. Pauli and Jules M. Blais and Vance L. Trudeau},
url = {https://www.frontiersin.org/articles/10.3389/fmars.2019.00533},
doi = {10.3389/fmars.2019.00533},
issn = {2296-7745},
year = {2019},
date = {2019-08-28},
urldate = {2019-01-01},
journal = {Frontiers in Marine Science},
volume = {6},
pages = {533},
abstract = {Naphthenic acids (NAs) are oil-derived mixtures of carboxylic acids and are considered emerging contaminants with the potential to disrupt development of aquatic species. In the Oil Sands Region of Canada, NAs are components of the water released following processing of the bitumen-containing sand. The aim of this research was to identify potential mechanisms of toxicity of NA mixtures. Silurana (Xenopus) tropicalis embryos were raised in water spiked with commercial oil-derived NA extracts (S1 and S2) at a sub-lethal concentration (2 mg/L). The transcriptomic responses of the whole 4-day old embryos following exposure were assessed using a custom oligonucleotide microarray. Both NA mixtures induced embryonic abnormalities that included edema, and cardiac and gut abnormalities. Exposure to NAs also affected morphometric parameters and decreased total length, tail length, and interorbital distance of the embryos. Gene ontology analysis revealed that 18 biological processes, 5 cellular components, and 19 molecular functions were significantly enriched after both S1 and S2 exposures. Sub-network enrichment analysis revealed pathways that were related to phenotypic abnormalities; these included gut function, edema, and cartilage differentiation. Other notable networks affected by NAs included metabolism and cell membrane integrity. In a separate dose-response experiment, the expression of key genes identified by microarray (cyp4b1, abcg2, slc26a6, eprs, and slc5a1) was determined by Real-Time qPCR in S. tropicalis embryos exposed to the commercial NAs and to acid-extractable organics (AEOs) prepared from Oil Sands Process-Affected Water. In general, the RT-qPCR data agreed with the microarray data. In S. tropicalis embryos exposed to the AEOs, the mRNA levels of eprs (bifunctional glutamate/proline-tRNA ligase) and slcs5a1 (sodium/glucose cotransporter 1) were significantly decreased compared to the controls. Such changes are likely indicative of increased edema and disrupted gut function, respectively. These data suggest that NAs have multiple modes of action to induce developmental toxicity in amphibians. Some modes of action may be shared between commercial NAs and AEOs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2018
Fonte D F D; Xing L; Mikwar M; Trudeau V L
In: Gen Comp Endocrinol, vol. 257, pp. 106–112, 2018, ISSN: 1095-6840.
@article{pmid28487180,
title = {Secretoneurin-A inhibits aromatase B (cyp19a1b) expression in female goldfish (Carassius auratus) radial glial cells},
author = {Dillon F Da Fonte and Lei Xing and Myy Mikwar and Vance L Trudeau},
doi = {10.1016/j.ygcen.2017.04.014},
issn = {1095-6840},
year = {2018},
date = {2018-02-01},
journal = {Gen Comp Endocrinol},
volume = {257},
pages = {106--112},
abstract = {In the teleost brain, radial glial cells (RGCs) are the main macroglia and are stem-like progenitors that express key steroidogenic enzymes, including the estrogen-synthesizing enzyme, aromatase B (cyp19a1b). As a result, RGCs are integral to neurogenesis and neurosteroidogenesis, however little is known about the regulatory factors and signaling mechanisms that control these functions. A potential new role of the secretogranin II-derived neuropeptide secretoneurin A (SNa) in the control of goldfish (Carassius auratus) RGC function is the subject of this study. Immunohistochemistry revealed a close neuroanatomical relationship between RGCs and soma of SNa-immunoreactive magnocellular and parvocellular neurons in the preoptic nucleus of female goldfish. Five hours following intracerebroventricular injection of 0.2ng/g SNa cyp19a1b mRNA levels were decreased by 86% (P<0.05) in the hypothalamus and by 88% (P<0.05) in the telencephalon. In vitro, 24 h incubation with 500nM SNa decreased cyp19a1b mRNA by 51% (P<0.05) in cultured RGCs. These data provide evidence that SNa can regulate aromatase expression in goldfish RGCs. By regulating neuroestrogen production in RGCs SNa may therefore be implicated in the control of major estrogen-dependent functions of the preoptic region such as reproductive behavior and osmoregulation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Fonte D F D; Martyniuk C J; Xing L; Trudeau V L
Secretoneurin A Directly Regulates the Proteome of Goldfish Radial Glial Cells
In: Front Endocrinol (Lausanne), vol. 9, pp. 68, 2018, ISSN: 1664-2392.
@article{pmid29559953,
title = {Secretoneurin A Directly Regulates the Proteome of Goldfish Radial Glial Cells },
author = {Dillon F Da Fonte and Chris J Martyniuk and Lei Xing and Vance L Trudeau},
doi = {10.3389/fendo.2018.00068},
issn = {1664-2392},
year = {2018},
date = {2018-01-01},
journal = {Front Endocrinol (Lausanne)},
volume = {9},
pages = {68},
abstract = {Radial glial cells (RGCs) are the main macroglia in the teleost brain and have established roles in neurogenesis and neurosteroidogenesis. They are the only brain cell type expressing aromatase B (), the enzyme that synthesizes estrogens from androgen precursors. There are few studies on the regulation of RGC functions, but our previous investigations demonstrated that dopamine stimulates expression in goldfish RGCs, while secretoneurin A (SNa) inhibits the expression of this enzyme. Here, we determine the range of proteins and cellular processes responsive to SNa treatments in these steroidogenic cells. The focus here is on SNa, because this peptide is derived from selective processing of secretogranin II in magnocellular cells embedded within the RGC-rich preoptic nucleus. Primary cultures of RGCs were treated (24 h) with 10, 100, or 1,000 nM SNa. By using isobaric tagging for relative and absolute quantitation and a Hybrid Quadrupole Obritrap Mass Spectrometry system, a total of 1,363 unique proteins were identified in RGCs, and 609 proteins were significantly regulated by SNa at one or more concentrations. Proteins that showed differential expression with all three concentrations of SNa included H1 histone, glutamyl-prolyl-tRNA synthetase, Rho GDP dissociation inhibitor γ, vimentin A2, and small nuclear ribonucleoprotein-associated protein. At 10, 100, and 1,000 nM SNa, there were 5, 195, and 489 proteins that were downregulated, respectively, whereas the number of upregulated proteins were 72, 44, and 51, respectively. Subnetwork enrichment analysis of differentially regulated proteins revealed that processes such as actin organization, cytoskeleton organization and biogenesis, apoptosis, mRNA processing, RNA splicing, translation, cell growth, and proliferation are regulated by SNa based on the proteomic response. Moreover, we observed that, at the low concentration of SNa, there was an increase in the abundance of proteins involved in cell growth, proliferation, and migration, whereas higher concentration of SNa appeared to downregulate proteins involved in these processes, indicating a dose-dependent proteome response. At the highest concentration of SNa, proteins linked to the etiology of diseases of the central nervous system (brain injuries, Alzheimer disease, Parkinson's disease, cerebral infraction, brain ischemia) were also differentially regulated. These data implicate SNa in the control of cell proliferation and neurogenesis.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Venables M J; Xing L; Edington C C; Trudeau V L
In: FACETS, vol. 3, no. 1, pp. 358-374, 2018, ISSN: 2371-1671.
@article{doi:10.1139/facets-2017-0119,
title = {Neuronal regeneration in the goldfish telencephalon following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) insult},
author = {Maddie J. Venables and Lei Xing and Connor C. Edington and Vance L. Trudeau},
url = {https://doi.org/10.1139/facets-2017-0119},
doi = {10.1139/facets-2017-0119},
issn = {2371-1671},
year = {2018},
date = {2018-01-01},
urldate = {2018-01-01},
journal = {FACETS},
volume = {3},
number = {1},
pages = {358-374},
abstract = {The constitutive regenerative ability of the goldfish central nervous system makes them an excellent model organism to study neurogenesis. Intraperitoneal injection of neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was used to deplete tyrosine hydroxylase-positive neurons in the adult goldfish telencephalon. We report novel information on the ability of the goldfish to regenerate (∼3–4 d post-MPTP insult) damaged neurons in telencephalic tissue by observing the rapid incorporation of bromodeoxyuridine into newly generated cells, which precedes the recovery of motor function in MPTP-treated animals. Specifically, the telencephalon area telencephali pars dorsalis in female goldfish, which is associated with fish motor activity, regenerates following MPTP toxicity. The remarkable ability of goldfish to rapidly regenerate damaged neurons provides insight into their use as model organisms to study neuroregenerative abilities within a few days following injury. We provide evidence that goldfish are able to regenerate neurons in ∼3–4 d to both replenish and recover baseline catecholaminergic levels, thus enabling the fish to reestablish basic activities such as swimming. The study of neuron regeneration in the damaged goldfish brain will increase our understanding of vertebrate neurogenesis and regeneration processes following central nervous system injury.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2017
Fonte D F D; Martyniuk C J; Xing L; Pelin A; Corradi N; Hu W; Trudeau V L
In: Sci Rep, vol. 7, no. 1, pp. 14930, 2017, ISSN: 2045-2322.
@article{pmid29097753,
title = {Secretoneurin A regulates neurogenic and inflammatory transcriptional networks in goldfish (Carassius auratus) radial glia},
author = {Dillon F Da Fonte and Christopher J Martyniuk and Lei Xing and Adrian Pelin and Nicolas Corradi and Wei Hu and Vance L Trudeau},
doi = {10.1038/s41598-017-14930-8},
issn = {2045-2322},
year = {2017},
date = {2017-11-01},
journal = {Sci Rep},
volume = {7},
number = {1},
pages = {14930},
abstract = {Radial glial cells (RGCs) are the most abundant macroglia in the teleost brain and have established roles in neurogenesis and neurosteroidogenesis; however, their transcriptome remains uncharacterized, which limits functional understanding of this important cell type. Using cultured goldfish RGCs, RNA sequencing and de novo transcriptome assembly were performed, generating the first reference transcriptome for fish RGCs with 17,620 unique genes identified. These data revealed that RGCs express a diverse repertoire of receptors and signaling molecules, suggesting that RGCs may respond to and synthesize an array of hormones, peptides, cytokines, and growth factors. Building upon neuroanatomical data and studies investigating direct neuronal regulation of RGC physiology, differential gene expression analysis was conducted to identify transcriptional networks that are responsive to the conserved secretogranin II-derived neuropeptide secretoneurin A (SNa). Pathway analysis of the transcriptome indicated that cellular processes related to the central nervous system (e.g., neurogenesis, synaptic plasticity, glial cell development) and immune functions (e.g., immune system activation, leukocyte function, macrophage response) were preferentially modulated by SNa. These data reveal an array of new functions that are proposed to be critical to neuronal-glial interactions through the mediator SNa.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xing L; Venables M J; Trudeau V L
In: Gen Comp Endocrinol, vol. 241, pp. 69–79, 2017, ISSN: 1095-6840.
@article{pmid26873632,
title = {Role of aromatase and radial glial cells in neurotoxin-induced dopamine neuron degeneration and regeneration},
author = {Lei Xing and Maddie J Venables and Vance L Trudeau},
doi = {10.1016/j.ygcen.2016.02.011},
issn = {1095-6840},
year = {2017},
date = {2017-01-01},
journal = {Gen Comp Endocrinol},
volume = {241},
pages = {69--79},
abstract = {Radial glial cells (RGCs) in teleost brain are progenitor cells that express aromatase B and produce estrogens. Controversial data suggest that estrogens are critical for brain repair and neurogenesis in teleosts. Using a goldfish model for neurotoxin-induced Parkinson-like syndrome, we investigated the possible roles of RGCs, especially estrogen synthetic function, in the processes underlying dopamine neuron regeneration. The data indicate that dopamine neuron degeneration and aromatase activity inhibition could be respectively achieved in vivo with treatments with the neurotoxin 1-methyl-1,2,3,6-tetrahydropyridine (MPTP) and fadrozole in female goldfish. The expression of genes in the telencephalon and hypothalamus related to RGC functions including gfap, gdnf and bdnf as well as genes related to mature dopamine neuron functions including th, slc6a3 and pitx3 are under modulation of estrogens. Together these results revealed that the activation of radial glial cells and dopamine neuron recovery after MPTP insult is aromatase-dependent. Findings in this study provide support for the hypothesis that endogenous estrogens are neuroprotective in goldfish. Future studies focus on the molecular pathways for enhancing protective functions of estrogens and understanding global effects of estrogens in the central nervous system.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2016
Xing L; Gutierrez-Villagomez J M; Fonte D F D; Venables M J; Trudeau V L
Dehydroabietic acid cytotoxicity in goldfish radial glial cells in vitro
In: Aquat Toxicol, vol. 180, pp. 78–83, 2016, ISSN: 1879-1514.
@article{pmid27658224,
title = {Dehydroabietic acid cytotoxicity in goldfish radial glial cells in vitro},
author = {Lei Xing and Juan Manuel Gutierrez-Villagomez and Dillon F Da Fonte and Maddie J Venables and Vance L Trudeau},
doi = {10.1016/j.aquatox.2016.09.009},
issn = {1879-1514},
year = {2016},
date = {2016-11-01},
journal = {Aquat Toxicol},
volume = {180},
pages = {78--83},
abstract = {Dehydroabietic acid (DHAA) is a resin acid present in aquatic environments shown to induce cellular and molecular damage in aquatic animals. In this study, the cytotoxicity of DHAA on primary cultured goldfish radial glial cells (RGCs), an important component of the central nervous system, was evaluated. Here, it is reported that a concentration of 20mg/L DHAA affected cellular morphology and expression of genes involved in RGC steroidogenesis and metabolism. Higher concentration exposures of DHAA (40mg/L) lead to RGC death based on a lactate dehydrogenase leakage assay. Together, these data have implications in understanding the effects of DHAA on an integral central nervous system cell type important for neurogenesis, steroidogenesis and structural support. Due to the continuous presence of DHAA into water systems, results from this study provide indications as to the potential impacts of DHAA and demonstrate the importance of this class of chemicals on aquatic organisms.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xing L; Martyniuk C J; Esau C; Fonte D F D; Trudeau V L
In: J Proteomics, vol. 144, pp. 123–132, 2016, ISSN: 1876-7737.
@article{pmid27185549,
title = {Proteomic profiling reveals dopaminergic regulation of progenitor cell functions of goldfish radial glial cells in vitro},
author = {Lei Xing and Christopher J Martyniuk and Crystal Esau and Dillon F Da Fonte and Vance L Trudeau},
doi = {10.1016/j.jprot.2016.05.003},
issn = {1876-7737},
year = {2016},
date = {2016-07-01},
urldate = {2016-07-01},
journal = {J Proteomics},
volume = {144},
pages = {123--132},
abstract = {Radial glial cells (RGCs) are stem-like cells found in the developing and adult central nervous system. They function as both a scaffold to guide neuron migration and as progenitor cells that support neurogenesis. Our previous study revealed a close anatomical relationship between dopamine neurons and RGCs in the telencephalon of female goldfish. In this study, label-free proteomics was used to identify the proteins in a primary RGC culture and to determine the proteome response to the selective dopamine D1 receptor agonist SKF 38393 (10μM), in order to better understand dopaminergic regulation of RGCs. A total of 689 unique proteins were identified in the RGCs and these were classified into biological and pathological pathways. Proteins such as nucleolin (6.9-fold) and ependymin related protein 1 (4.9-fold) were increased in abundance while proteins triosephosphate isomerase (10-fold) and phosphoglycerate dehydrogenase (5-fold) were decreased in abundance. Pathway analysis revealed that proteins that consistently changed in abundance across biological replicates were related to small molecules such as ATP, lipids and steroids, hormones, glucose, cyclic AMP and Ca(2+). Sub-network enrichment analysis suggested that estrogen receptor signaling, among other transcription factors, is regulated by D1 receptor activation. This suggests that these signaling pathways are correlated to dopaminergic regulation of radial glial cell functions. Most proteins down-regulated by SKF 38393 were involved in cell cycle/proliferation, growth, death, and survival, which suggests that dopamine inhibits the progenitor-related processes of radial glial cells. Examples of differently expressed proteins including triosephosphate isomerase, nucleolin, phosphoglycerate dehydrogenase and capping protein (actin filament) muscle Z-line beta were validated by qPCR and western blot, which were consistent with MS/MS data in the direction of change. This is the first study to characterize the RGC proteome on a large scale in a vertebrate species. These data provide novel insight into glial protein networks that are associated with neuroendocrine function and neurogenesis in the teleost brain.nnBIOLOGICAL SIGNIFICANCE: While the role of radial glial cells in organizing brain structure and neurogenesis has been well studied, protein profiling experiments in this unique cell type has not been conducted. This study is the first to profile the proteome of goldfish radial glial cells in culture and to study the regulation of progenitor functions of radial glial cells by the neurotransmitter dopamine. This study provides the foundation for molecular network analysis in fish radial glial cells, and identifies cellular processes and signaling pathways in these cells with roles in neurogenesis and neuroendocrine function. Lastly, this study begins to characterize signatures and biomarkers for specific neuroendocrine and neurogenesis disruptors.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mikwar M; Navarro-Martin L; Xing L; Volkoff H; Hu W; Trudeau V L
In: Peptides, vol. 78, pp. 42–50, 2016, ISSN: 1873-5169.
@article{pmid26860475,
title = {Stimulatory effect of the secretogranin-ll derived peptide secretoneurin on food intake and locomotion in female goldfish (Carassius auratus)},
author = {M Mikwar and L Navarro-Martin and Lei Xing and H Volkoff and W Hu and Vance L Trudeau},
doi = {10.1016/j.peptides.2016.01.007},
issn = {1873-5169},
year = {2016},
date = {2016-04-01},
urldate = {2016-04-01},
journal = {Peptides},
volume = {78},
pages = {42--50},
abstract = {Secretoneurin (SN) is a conserved peptide derived by proteolytic processing from the middle domain of the ∼600 amino acid precursor secretogranin-II (SgII). Secretoneurin is widely distributed in secretory granules of endocrine cells and neurons and has important roles in reproduction as it stimulates luteinizing hormone release from the pituitary. A potential new role of SN in goldfish feeding is the subject of this study. Firstly, we established that acute (26 h; p<0.0001) and short-term (72 h; p=0.016) fasting increased SgIIa precursor mRNA levels 1.25-fold in the telencephalon, implicating SN in the control of feeding. Secondly, we determined that intracerebroventricular injections of the type A SN (SNa; 0.2 and 1 ng/g BW) increased food intake and locomotor behavior by 60 min. Fish injected with the lower and higher doses of SNa (0.2 and 1 ng/g) respectively exhibited significant 1.77- and 2.58-fold higher food intake (p<0.0001) than the saline-injected control fish. Locomotor behavior was increased by 1.35- and 2.26-fold for 0.2 ng/g SNa (p=0.0001) and 1 ng/g SNa (p<0.0001), respectively. Injection of 1 ng/g SNa increased mRNA levels of hypothalamic neuropeptide Y 1.36-fold (p=0.038) and decreased hypothalamic cocaine-and amphetamine-regulated transcript by 33% (p=0.01) at 2h and 5h post-injection, respectively. These data suggest interactions of SNa with stimulatory and inhibitory pathways of food intake control in fish.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2015
Xing L; Esau C; Trudeau V L
In: Front Neurosci, vol. 9, pp. 504, 2015, ISSN: 1662-4548.
@article{pmid26793050,
title = {Direct Regulation of Aromatase B Expression by 17β-Estradiol and Dopamine D1 Receptor Agonist in Adult Radial Glial Cells},
author = {Lei Xing and Crystal Esau and Vance L Trudeau},
doi = {10.3389/fnins.2015.00504},
issn = {1662-4548},
year = {2015},
date = {2015-01-01},
journal = {Front Neurosci},
volume = {9},
pages = {504},
abstract = {Aromatase cytochrome P450arom (cyp19) is the only enzyme that has the ability to convert androgens into estrogens. Estrogens, which are produced locally in the vertebrate brain play many fundamental roles in neuroendocrine functions, reproductive functions, socio-sexual behaviors, and neurogenesis. Radial glial cells (RGCs) are neuronal progenitor cells that are abundant in fish brains and are the exclusive site of aromatase B expression and neuroestrogen synthesis. Using a novel in vitro RGC culture preparation we studied the regulation of aromatase B by 17β-estradiol (E2) and dopamine (DA). We have established that activation of the dopamine D1 receptor (D1R) by SKF 38393 up-regulates aromatase B gene expression most likely through the phosphorylation of cyclic AMP response element binding protein (CREB). This up-regulation can be enhanced by low concentration of E2 (100 nM) through increasing the expression of D1R and the level of p-CREB protein. However, a high concentration of E2 (1 μM) and D1R agonist together failed to up-regulate aromatase B, potentially due to attenuation of esr2b expression and p-CREB levels. Furthermore, we found the up-regulation of aromatase B by E2 and DA both requires the involvement of esr1 and esr2a. The combined effect of E2 and DA agonist indicates that aromatase B in the adult teleost brain is under tight control by both steroids and neurotransmitters to precisely regulate neuroestrogen levels.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xing L; McDonald H; Fonte D F D; Gutierrez-Villagomez J M; Trudeau V L
In: Front Neurosci, vol. 9, pp. 310, 2015, ISSN: 1662-4548.
@article{pmid26388722,
title = {Dopamine D1 receptor activation regulates the expression of the estrogen synthesis gene aromatase B in radial glial cells},
author = {Lei Xing and Heather McDonald and Dillon F Da Fonte and Juan M Gutierrez-Villagomez and Vance L Trudeau},
doi = {10.3389/fnins.2015.00310},
issn = {1662-4548},
year = {2015},
date = {2015-01-01},
journal = {Front Neurosci},
volume = {9},
pages = {310},
abstract = {Radial glial cells (RGCs) are abundant stem-like non-neuronal progenitors that are important for adult neurogenesis and brain repair, yet little is known about their regulation by neurotransmitters. Here we provide evidence for neuronal-glial interactions via a novel role for dopamine to stimulate RGC function. Goldfish were chosen as the model organism due to the abundance of RGCs and regenerative abilities of the adult central nervous system. A close anatomical relationship was observed between tyrosine hydroxylase-positive catecholaminergic cell bodies and axons and dopamine-D1 receptor expressing RGCs along the ventricular surface of telencephalon, a site of active neurogenesis. A primary cell culture model was established and immunofluorescence analysis indicates that in vitro RGCs from female goldfish retain their major characteristics in vivo, including expression of glial fibrillary acidic protein and brain lipid binding protein. The estrogen synthesis enzyme aromatase B is exclusively found in RGCs, but this is lost as cells differentiate to neurons and other glial types in adult teleost brain. Pharmacological experiments using the cultured RGCs established that specific activation of dopamine D1 receptors up-regulates aromatase B mRNA through a cyclic adenosine monophosphate-dependent molecular mechanism. These data indicate that dopamine enhances the steroidogenic function of this neuronal progenitor cell.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2014
Xing L; Goswami M; Trudeau V L
Radial glial cell: Critical functions and new perspective as a steroid synthetic cell
In: General and comparative endocrinology, vol. 203, pp. 181-185, 2014, ISSN: 0016-6480.
@article{XingL2014RgcC,
title = {Radial glial cell: Critical functions and new perspective as a steroid synthetic cell},
author = {Lei Xing and M Goswami and Vance L Trudeau},
doi = {10.1016/j.ygcen.2014.03.010 },
issn = {0016-6480},
year = {2014},
date = {2014-07-01},
urldate = {2014-07-01},
journal = {General and comparative endocrinology},
volume = {203},
pages = {181-185},
publisher = {Elsevier Inc},
address = {United States},
abstract = {The radial glial cell (RGC) is a glial cell type in the central nervous system of all vertebrates. Adult teleost fish have abundant RGCs in the brain in contrast to mammals. Adult fish RGCs have many important functions, including forming a structural scaffold to guide neuronal migration and serving as the progenitor cells in the brain to generate neurons. The role of the RGC in adult neurogenesis explains the high regenerative capacity of adult fish brain. There is increasing evidence from several species that some glial cells produce or metabolize steroids. It is now well-known that teleost RGCs express aromatase and produce estrogens from androgen precursors, which may be important for local neuroendocrine functions and regulation of neurogenesis. The question of whether RGCs are capable of de novo steroid synthesis from cholesterol remains unanswered. However, the expression of steroidogenic acute regulatory protein, and the key enzyme cytochrome P450 17alpha-hydroxylase in primary cultures of goldfish RGCs indicate the potential to produce 17α-hydroxy-pregnenolone and thus other steroid intermediates. The possibility of synthesizing additional non-estrogenic steroids may indicate new functions for the RGC.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2012
Wang Q; Yang X; Ren M; Hu Y; Chen Q; Xing L; Meng C; Liu T
Effect of chitosan/type I collagen/gelatin composites in biocompatibility and nerve repair
In: Neural regeneration research, vol. 7, no. 15, pp. 1179-1184, 2012, ISSN: 1673-5374.
@article{WangQing2012EocI,
title = {Effect of chitosan/type I collagen/gelatin composites in biocompatibility and nerve repair},
author = {Qing Wang and Xiaolei Yang and Ming Ren and Yulin Hu and Qiang Chen and Lei Xing and Chunyang Meng and Tiemei Liu},
doi = {10.3969/j.issn.1673-5374.2012.15.009},
issn = {1673-5374},
year = {2012},
date = {2012-05-25},
urldate = {2012-01-01},
journal = {Neural regeneration research},
volume = {7},
number = {15},
pages = {1179-1184},
publisher = {China-Japan Union Hospital, Jilin University, Changchun 130033, Jilin Province, China%Department of Toxicology, School of Public Health, Jilin University, Changchun 130021, Jilin Province, China%Department of Liver, Gall and Pancreas, First Hospital of Jilin University, Changchun 130021, Jilin Province, China},
address = {India},
abstract = {Chitosan, collagen I and gelatin were mixed in appropriate quantities to develop a new nerve repair material, with good arrangement and structure, as well as even aperture size. The composite material was sterilized by (60)Co irradiation for 24 hours prior to implantation in the right thigh of rats following sciatic nerve damage. Results showed that the material was nontoxic to the kidneys and the liver, and did not induce an inflammatory response in the muscles. The composite material enhanced the recovery of sciatic nerve damage in rats. These experimental findings indicate that the composite material offers good biocompatibility and has a positive effect on injured nerve rehabilitation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}